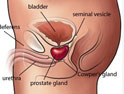
Castration-resistant prostate cancer is a fatal form of prostate cancer, the prognosis is poor, with a median survival time of 12 to 18 months. China is still a lack of effective means of treatment of CRPC. In this paper, bicalutamide treatment CRPC reviewed relevant research that bicalutamide especially in large doses, is CRPC effective treatment strategies for domestic CRPC treatment of patients has important clinical significance. However, the specific effect remains to be further studied.
 Astellas US LLC, a United States (U.S.) subsidiary of Tokyo-based Astellas Pharma Inc. (TSE: 4503), and Medivation, Inc. (Nasdaq: MDVN) today announced that results from the STRIVE trial of enzalutamide compared to bicalutamide in men with castration-resistant prostate cancer (CRPC) were published in the Journal of Clinical Oncology. The article, titled, “Enzalutamide Versus Bicalutamide in Castration-Resistant Prostate Cancer: The STRIVE Trial,” appears in the January 25, 2016 online issue and will be published in a future print issue of the journal.
Astellas US LLC, a United States (U.S.) subsidiary of Tokyo-based Astellas Pharma Inc. (TSE: 4503), and Medivation, Inc. (Nasdaq: MDVN) today announced that results from the STRIVE trial of enzalutamide compared to bicalutamide in men with castration-resistant prostate cancer (CRPC) were published in the Journal of Clinical Oncology. The article, titled, “Enzalutamide Versus Bicalutamide in Castration-Resistant Prostate Cancer: The STRIVE Trial,” appears in the January 25, 2016 online issue and will be published in a future print issue of the journal.
The study achieved its primary endpoint demonstrating a statistically significant increase in progression-free survival (PFS) for enzalutamide compared with bicalutamide (Hazard Ratio = 0.24; 95% Confidence Interval, 0.18-0.32; p<0.0001). Median PFS was 19.4 months in the enzalutamide group compared with 5.7 months in the bicalutamide group.
The median time on treatment in the STRIVE trial was 14.7 months in the enzalutamide group versus 8.4 months in the bicalutamide group. Serious adverse events were reported in 29.4% of enzalutamide-treated patients and 28.3% of bicalutamide-treated patients. Grade 3 or higher cardiac adverse events were reported in 5.1% of enzalutamide-treated patients versus 4.0% of bicalutamide-treated patients. One seizure was reported in the trial in the enzalutamide-treated group and none in the bicalutamide-treated group. The most common side effects noted more frequently in the enzalutamide-treated versus bicalutamide-treated patients included fatigue, back pain, hot flush, fall, hypertension, dizziness, and decreased appetite, consistent with the known safety profile of enzalutamide.
The STRIVE study is the second of two head-to-head studies of enzalutamide versus bicalutamide, the first of which was TERRAIN, which was published in the January 13, 2016 online issue of Lancet Oncology.