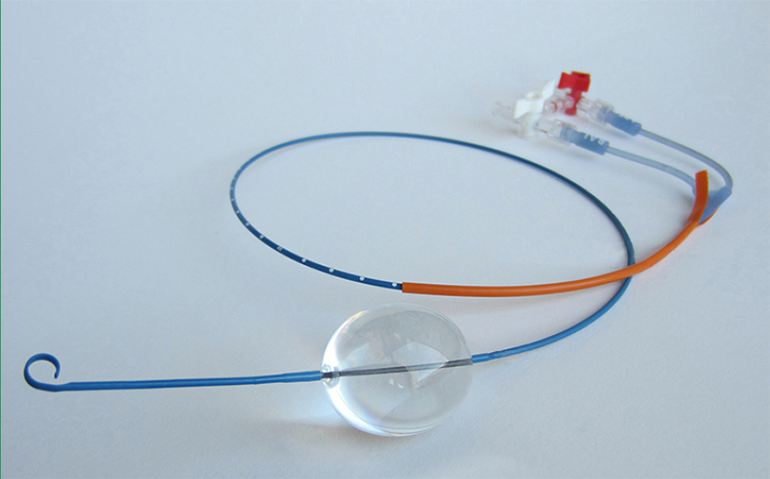
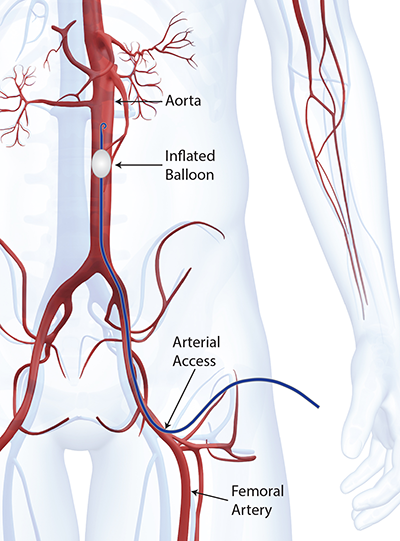
 Xconomy is reporting that Prytime Medical Devices, a company based outside of San Antonio, Texas formerly known as Pryor Medical Devices, won CE Mark clearance to introduce its ER-REBOA emergency occlusion balloon catheter in Europe. Designed to occlude large vessels, the aorta in particular, it provides time for doctors to stabilize a patient before too much blood loss occurs.
Xconomy is reporting that Prytime Medical Devices, a company based outside of San Antonio, Texas formerly known as Pryor Medical Devices, won CE Mark clearance to introduce its ER-REBOA emergency occlusion balloon catheter in Europe. Designed to occlude large vessels, the aorta in particular, it provides time for doctors to stabilize a patient before too much blood loss occurs.
To speed things up, it doesn’t require a fluoroscope to position the balloon and no wire exchanges are performed, as in many other minimally invasive procedures. It has a soft tip, is sized for 7 French sheeths, and has an integrated arterial pressure monitoring sensor built-in to keep a continuous eye on the status of the patient.
The device was cleared by the FDA in the U.S. in the fall of 2015.
Flashbacks: ER-REBOA Catheter for Emergency Occlusion of Aorta FDA Cleared…; Pryor Medical Devices Wins $14.3MM Contract for REBOA Research…
Product page: ER-REBOA…
Via: Xconomy…



